The EU healthcare and MedTech sectors are under the sharpest regulatory spotlight they’ve ever faced. Rules that once sounded distant like the AI Act, MDR / IVDR, and EU Clinical Trials Regulation (CTR) are now taking effect, revealing a common thread: language is compliance. From AI-driven diagnostics to implant labeling and public trial data, every translated word can determine whether a product passes audit, enters the market, or ends up delayed by months.
Below are the three biggest localisation challenges facing European healthcare and MedTech companies right now and recommendation on how a compliance-ready translation process turns each one from a risk into a competitive edge.
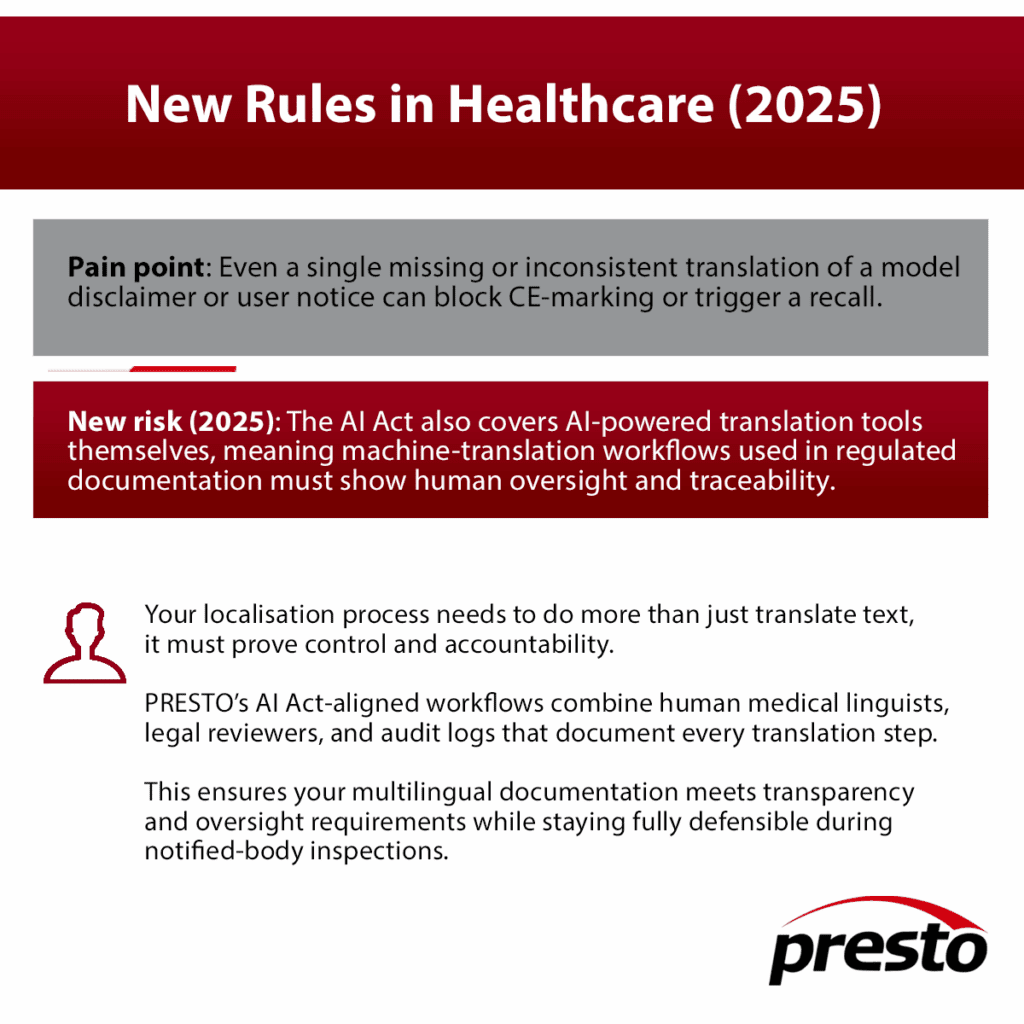
1. Your multilingual documentation will be audited due to EU AI Act
The AI Act, adopted in 2024 and now fully in force, classifies most medical-device and clinical decision-support AI systems as “high-risk.” Between 2025 and 2027, manufacturers and software providers must meet strict requirements for:
- Transparent system documentation and risk summaries
- Human-oversight instructions
- Logs and model-change records
- Clear explanations of algorithmic logic for end users
Each of these elements must be available in the official languages of the Member States where the product is placed on the market.
2. Device labeling & language-table updates — “English-only” is no longer safe
Under the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR), every product must supply Instructions for Use (IFU), labels, and safety information in the language(s) officially required by each Member State.
In August 2024, the European Commission published an updated language-requirements table confirming that several countries tightened their national rules. Markets that once accepted English-only documentation (for professional-use devices) now demand full local-language IFUs or patient leaflets. Many manufacturers still rely on outdated tables or assume a “one-size-fits-all” English IFU. That’s now a regulatory risk.
PRESTO helps you stay compliant with every EU country’s current language obligations through an updated 2025 MDR/IVDR matrix and smart optimisation of your existing content. We streamline your IFUs, labels, and safety information so they meet national requirements without inflating translation volume or production time. The result is consistent, compliant documentation ready for any EU market.
3. Multilingual communication builds trust thanks to clinical trials & public transparency
Since early 2025, all active trials have transitioned into the EU Clinical Trials Information System (CTIS) under the Clinical Trials Regulation (CTR 536/2014). Lay summaries and study details are now publicly visible, and ethics committees increasingly expect local-language recruitment and consent materials to ensure participant understanding. Poorly translated consent forms or participant materials can cause ethics-committee delays, patient drop-outs, or reputational damage once summaries appear online.
PRESTO provides complete multilingual support for clinical-trial communication, from participant recruitment to consent forms and lay summaries. Our linguists adapt every text to local cultural and literacy standards, ensuring patients clearly understand procedures and risks. With readability testing, back-translation, and version control, your materials are ready for ethics-committee approval and public release through CTIS.
Why this matters now
Across all three fronts, the visibility of language has exploded. AI systems must “explain themselves” in every EU language, device packaging must reflect updated national tables, and clinical data must be readable to the public.
For healthcare and MedTech companies, localisation is now part of your regulatory and quality infrastructure.
How PRESTO helps
We build audited localisation workflows that meet regulatory, linguistic, and patient-safety expectations.
Our in-house medical linguists, legal reviewers, and AI-compliance specialists deliver:
- MDR / IVDR / AI Act-aligned translation processes
- Terminology consistency across documentation sets
- Full traceability and versioning for notified-body or authority review
Free EU compliance localisation audit
Do you need to identify where your multilingual documentation could expose you to risk — from AI Act disclosures to MDR labels and CTIS materials?
We offer a concise action plan within 48 hours, showing your biggest compliance gaps and quick-fix priorities. You’ll receive actionable insight to secure your EU market access with no commitment.
Key official sources for the major EU healthcare/localisation-relevant regulations:
- Regulation (EU) 2024/1689 (Artificial Intelligence Act) — full text of the EU AI Act as published in the Official Journal
- Regulation (EU) No 536/2014 (Clinical Trials Regulation) — harmonises clinical trial procedures, transparency & multilingual requirements
- Regulation (EU) 2017/745 (Medical Device Regulation) & 2017/746 (IVDR) — includes national language‐requirements tables for device documentation (IFUs, labels)
